The simple definition of water hardness is the amount of dissolved calcium and magnesium in the water. Hard water is high in dissolved minerals, both calcium and magnesium. The water hardness is defined as the total hardness (TH) which primarily corresponds to calcium and magnesium salts. More the water is rich in calcium (limestone) and magnesium, more it is called "hard". Conversely a poor water in limestone is called "soft".
Hardness is caused by compounds of mainly calcium and magnesium, and by a variety of other metals: iron, aluminium, and manganese can also be present at elevated levels in some locations. General guidelines for classification of waters are: 0 to 60 mg/L (milligrams per liter) as calcium carbonate is classified as soft; 61 to 120 mg/L as moderately hard; 121 to 180 mg/L as hard; and more than 180 mg/L as very hard.
The water hardness is expressed by an index, or total hardness, TH, in degrees, each degree determined by the following formula:
1 degree (°F : French unit) = 4 mg/liter of calcium or 2.43 mg/liter of magnesium or 10 mg of limestone.
The waters are classified according to their TH (French TH)
0 to 6 degrees very soft water
6 to 15 degrees soft water
15 to 25 degrees moderately hard water
25 to 35 degrees hard water
> 35 degrees very hard water
Water hardness can be expressed in various units, such as degrees of general hardness (dGH), German degrees (°dH), parts per million (ppm, mg/L, or American degrees), grains per gallon (gpg), English degrees (°e, e, or °Clark), or French degrees (°fH, °f or °F; lowercase f is used to prevent confusion with degrees Fahrenheit). The table below shows conversion factors between the various units.
It is easy to detect, without testing, the hardness of your water :
With soft water (rain water), soap makes quickly foam and it is hard to get rid of the soap.
On the other hand, hard water (limestone) quickly removes soap and even gives hands a little rough after washing. But it needs more soap to wash or clean.
You may have felt the effects of hard water, literally, the last time you washed your hands. Depending on the hardness of your water, after using soap to wash you may have felt like there was a film of residue left on your hands. In hard water, soap reacts with the calcium (which is relatively high in hard water) to form "soap scum". When using hard water, more soap or detergent is needed to get things clean, be it your hands, hair, or your laundry.
 Home boiler resistor
Home boiler resistor  Scaling, limestone, rust and corrosion
Scaling, limestone, rust and corrosion
 Huge scaling, limestone, rust and corrosion
Huge scaling, limestone, rust and corrosion
 Alternance of soft/hard water and limestone
Alternance of soft/hard water and limestone
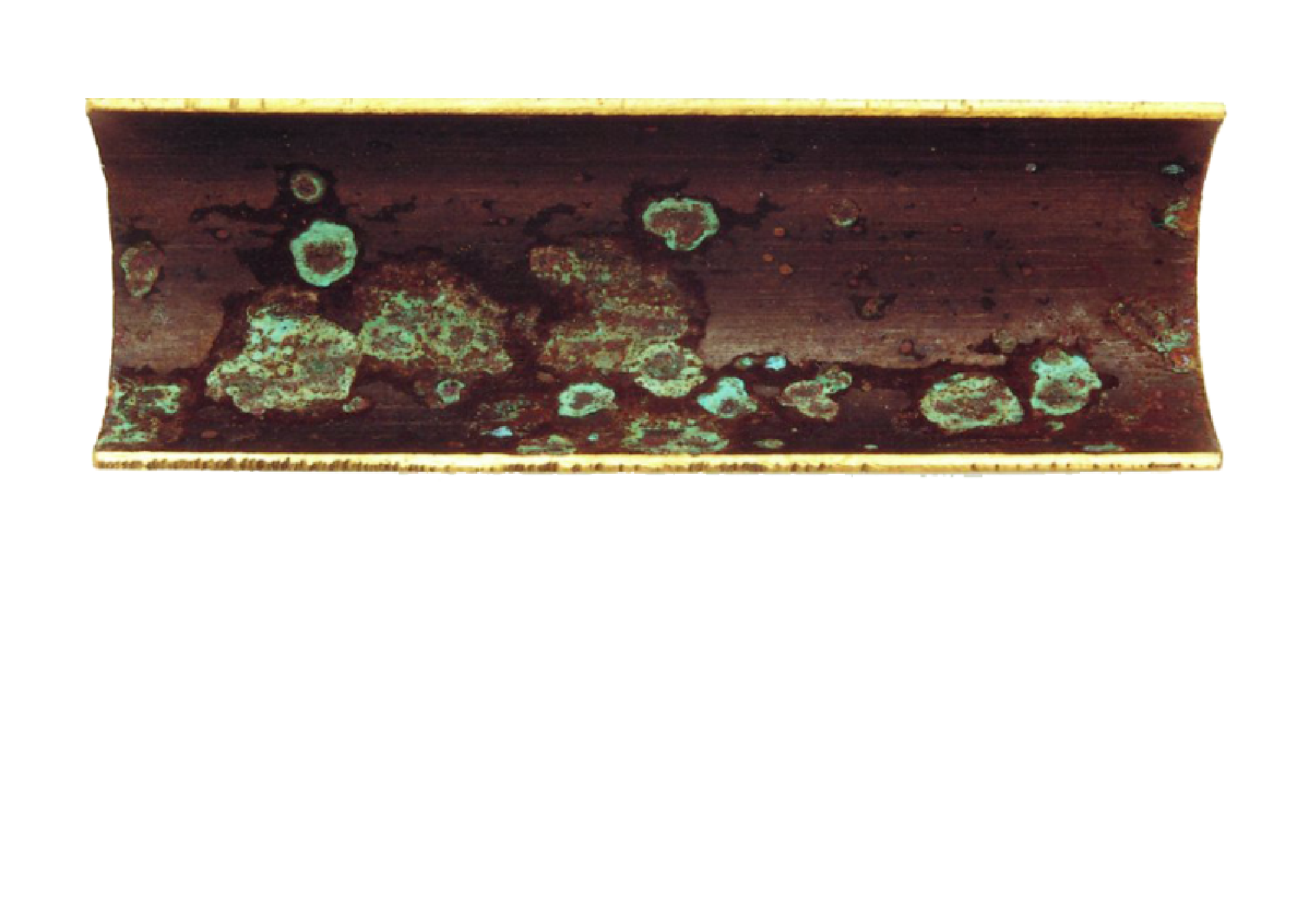
 copper corrosion
copper corrosion